Imagine having the power to rebuild damaged tissue, not just manage the pain. That is the promise of Stem Cell Therapy. This revolutionary approach in regenerative medicine is changing the game for chronic diseases, offering hope for true repair where conventional medicine often falls short. Read on to understand how your body’s own healing tools are being used to fight disease and promote tissue regeneration.
Key Takeaways
- What it is: Stem cell therapy uses specialized cells to repair damaged tissues, reduce inflammation, and regenerate organs by leveraging their unique ability to differentiate and self-renew.
- Current success rates: Clinical trials show success rates ranging from 50% to 90% depending on the condition, with particularly strong outcomes in blood disorders (60-70%), joint repair (80%), and cardiac applications (65-79% reduction in major cardiovascular events).
- Main types: Mesenchymal stem cells (MSCs) from bone marrow or fat tissue are most commonly used in clinical practice, while induced pluripotent stem cells (iPSCs) and hematopoietic stem cells (HSCs) are expanding therapeutic options.
- FDA status: Hematopoietic stem cell transplantation is the only FDA-approved stem cell therapy for routine clinical use, though other applications are rapidly moving through clinical trials with encouraging safety profiles.
- Real-world outcomes: Patients report improved quality of life, reduced pain, enhanced mobility, and lower hospitalization rates within 3–6 months of stem cell treatment.
Understanding Stem Cell Therapy: What Is It Exactly?
Stem cell therapy is a cutting-edge approach in regenerative medicine that aims to repair or replace damaged tissue at the cellular level, moving beyond just managing symptoms. This cellular therapy taps into the body’s natural ability to heal by using specialized stem cells that can self-renew and transform into any cell type needed for tissue regeneration, such as nerve or cartilage cells. The therapy works through direct cell replacement and by releasing powerful healing compounds (known as paracrine signaling) that modulate the immune system and reduce harmful inflammation, offering a unique solution where traditional medicine falls short.
Types of Stem Cells Used in Clinical Practice
Not all stem cells are the same. The type of stem cell used influences the therapy’s potential applications, safety profile, and overall effectiveness. Understanding these differences helps explain why certain stem cell treatments are approved while others remain experimental.
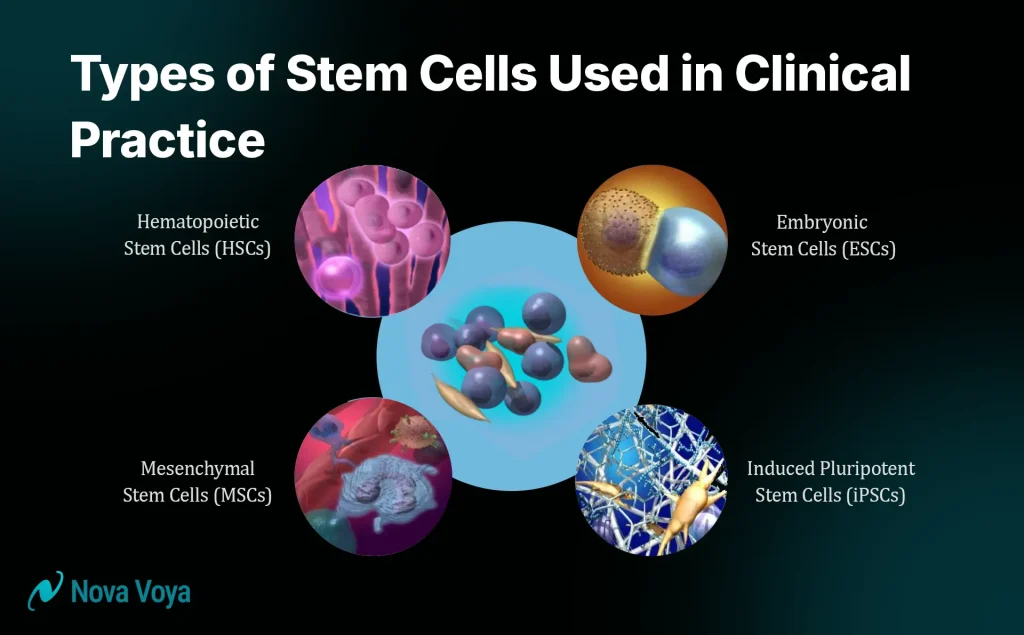
1. Mesenchymal Stem Cells (MSCs): The Workhorses of Regenerative Medicine
Mesenchymal stem cells (MSCs) are adult stem cells found mainly in bone marrow, fat (adipose) tissue, and umbilical cord tissue. They are the most widely used stem cells today in clinical practice and research for regenerative medicine.
Key characteristics of MSCs:
- Multipotent: They can differentiate into bone, cartilage, muscle, and fat cells.
- Low Immunogenicity: They can often be transplanted from donors (allogeneic) without causing severe immune rejection, simplifying the stem cell transplant process.
- Immunomodulatory properties: They actively reduce inflammation, making them ideal for autoimmune conditions.
- Easy to Isolate: They are relatively simple to isolate and expand in a lab to achieve therapeutic cell numbers.
Adipose-derived MSCs are popular because fat tissue contains significantly more stem cells per gram than bone marrow. This abundance makes harvesting fat tissue convenient (via a simple liposuction procedure) and yields higher cell counts, supporting widespread stem cell treatment in orthopedics.
2. Induced Pluripotent Stem Cells (iPSCs): The Next Generation of Stem Cell Therapy
Induced pluripotent stem cells (iPSCs) are specialized adult cells (like skin cells) that have been genetically reprogrammed to revert to an embryonic-like state. This breakthrough, which earned a Nobel Prize in 2012, allows scientists to “reset” a specialized cell.
How do they work?
Scientists introduce four specific genes (Yamanaka factors) into adult cells. This reprogramming converts the cell into a pluripotent state, meaning it is capable of becoming almost any cell type in the body, which is critical for specialized tissue regeneration.
Advantages of iPSCs: They can be created from the patient’s own cells, virtually eliminating rejection risk (autologous source). They offer a theoretically unlimited supply and can be genetically customized before differentiation into specific cells, such as dopamine-producing neurons for Parkinson’s disease.
As of late 2024, clinical trials involving iPSC-derived products show encouraging safety profiles, rapidly accelerating the future of this cellular therapy.
3. Hematopoietic Stem Cells (HSCs): Blood and Immune System Specialists
Hematopoietic stem cells (HSCs) are blood-forming stem cells that create all blood cell types and immune cells. These are the only stem cell-based therapies currently approved by the FDA for routine clinical use, primarily through stem cell transplant procedures.
Clinical applications of HSCs:
- Treating leukemias and lymphomas (via bone marrow transplantation).
- Managing blood disorders like sickle cell disease and aplastic anemia.
- Restoring immune function and preventing graft-versus-host disease (GVHD).
HSCT has a long clinical history with proven success, including impressive three-year survival rates of 92% for Hodgkin lymphoma and 79% for multiple myeloma patients.
4. Embryonic Stem Cells (ESCs): Limited Clinical Use
Embryonic stem cells (ESCs) are pluripotent cells derived from the early embryo. While they are highly potent and valuable for research, their clinical use remains limited due to ethical considerations and strict regulatory restrictions. Additionally, ESCs historically pose a higher risk of tumor formation (teratoma) when transplanted compared to the more mature MSCs and differentiated iPSCs.
How Stem Cell Therapy Works: The Mechanisms Behind Healing
Stem cell therapy works through multiple powerful biological mechanisms simultaneously, which is why this regenerative medicine approach is so effective for various complex diseases.
1. Differentiation: Direct Cell Replacement
Differentiation is the most direct action: replacing lost or damaged cells. When stem cells enter an injured area, they receive signals from the environment and transform into the specific cell type needed for repair, completing tissue regeneration. For example, when MSCs are injected into an arthritic joint, some may differentiate into chondrocytes (cartilage cells) to replace lost cartilage. In conditions like Parkinson’s disease, iPSCs can be programmed to become the necessary dopaminergic neurons.
2. Paracrine Signaling: The Communication Revolution
Paracrine signaling is often the primary driver of the effectiveness of stem cell treatment. The stem cells act as mobile pharmacies, releasing bioactive compounds that influence healing in surrounding tissues.
Stem cells secrete:
- Growth factors that promote tissue repair and new blood vessel formation.
- Cytokines that modulate inflammatory responses.
- Extracellular vesicles (like exosomes) that carry therapeutic molecules.
- Anti-inflammatory compounds that significantly reduce harmful inflammation.
In osteoarthritis, for instance, the stem cells reduce inflammation and create a healing microenvironment, promoting the joint’s natural repair processes. This explains why relief can be sustained even if cartilage regrowth is incomplete.
3. Immunomodulation: Rebalancing the Immune System
The immunomodulatory properties of MSCs are crucial, allowing them to sense and actively suppress excessive and damaging immune activity common in chronic diseases. They shift the immune system from a pro-inflammatory state to an anti-inflammatory state by suppressing aggressive T cells and promoting regulatory T cells. This function makes MSCs highly valuable for autoimmune diseases like multiple sclerosis and rheumatoid arthritis, offering a targeted alternative to systemic immunosuppressive drugs.
4. Angiogenesis and Tissue Support
Stem cells also stimulate the formation of new blood vessels (angiogenesis), which is essential for delivering oxygen and nutrients to healing tissues—a necessity for successful tissue regeneration. They further protect existing cells by reducing apoptosis (programmed cell death) and providing supportive factors, ensuring comprehensive cellular therapy.
Clinical Applications and Proven Results of Stem Cell Therapy
Stem cell therapy is showing measurable benefits across many medical conditions, with clinical results demonstrating strong outcomes in specific therapeutic areas.
1. Cardiac and Cardiovascular Disease
Stem cell therapy has shown remarkable promise in improving heart function in patients with advanced heart failure. Clinical trials highlight the benefits of this regenerative medicine approach:
- Patients receiving stem cells reported significant improvements in quality of life and physical function.
- They showed significantly lower hospitalization rates (10.6% vs 27.1% in conventional groups).
- They experienced a reduced risk of major cardiovascular events (65% reduction overall).
- A comparative study showed stem cells led to a 13.4 percentage point improvement in LVEF (a measure of heart function) compared to only 4.3 points in conventional therapy, and resulted in 2.7x greater improvement in exercise capacity.
2. Neurological Disorders
Neurological conditions like Parkinson’s disease, multiple sclerosis, and spinal cord injuries are major frontiers for stem cell therapy.
- Parkinson’s Disease: Recent trials using dopamine-producing neurons derived from stem cells show promising safety profiles, continued neuron survival 18 months after stem cell transplant, and visible reductions in tremors and improved motor function in some participants.
- Multiple Sclerosis (MS): HSCT has shown superior results compared to medication alone, leading to a 19% improvement in disability scores over five years and significant immune system rebalancing.
- Spinal Cord Injuries (SCI): Trials using adipose-derived MSCs reported that 7 out of 10 severe injury participants advanced at least one grade on the functional impairment scale, demonstrating real functional improvement.
3. Orthopedic and Joint Conditions
Stem cell therapy is a minimally invasive alternative to surgery for osteoarthritis and injuries. MSCs injected directly into a damaged joint work by releasing growth factors, reducing pro-inflammatory molecules, and promoting the joint’s natural repair response and tissue regeneration.
Crucially, success rates for joint repair and orthopedic applications using MSC therapy reach approximately 80% positive outcomes. Patients favor this stem cell treatment due to its minimally invasive nature and shorter recovery time compared to joint replacement surgery.
4. Blood Disorders and Hematologic Malignancies
This is the most established application of stem cell therapy, fully FDA-approved for hematopoietic stem cell transplantation (HSCT). It is the cornerstone of effective cellular therapy for conditions like leukemias, lymphomas, aplastic anemia, and sickle cell disease. Success rates are high, with three-year survival rates reaching 92% for Hodgkin lymphoma and 79% for multiple myeloma.
5. Autoimmune and Inflammatory Diseases
The strong immunomodulatory properties of MSCs make them ideal for conditions where the immune system is overactive, including rheumatoid arthritis, systemic lupus erythematosus (SLE), and Type 1 diabetes. Stem cell treatment works by promoting a shift to anti-inflammatory immune cell profiles, which is a targeted benefit over systemic immunosuppressive drugs.
6. Liver Disease
Stem cell therapy shows promise for liver conditions like cirrhosis. MSCs promote liver cell regeneration, reduce inflammation, and may help reverse some fibrotic changes associated with chronic liver disease, supporting tissue regeneration.
Stem Cell Therapy Success Rates: What the Data Shows
Clinical data confirms that stem cell treatment is highly effective, though rates vary by application:
- Blood Disorders/Hematopoietic: 60-70% success rate overall (72-92% three-year survival for specific cancers).
- Joint Repair/Orthopedic: Approximately 80% positive outcomes.
- Autoimmune/Inflammatory: 80% positive outcomes reported.
- Cardiac Applications: 50-65% success rate in reducing major cardiovascular events.
Meaningful improvements typically emerge within 3–6 months of stem cell treatment, reflecting the biological timeline of tissue regeneration.
Stem Cell Therapy: Administration Routes and Treatment Protocols
The method of stem cell delivery is chosen based on the patient’s condition:
- Intravenous (IV) Administration: Systemic delivery via the bloodstream; useful for widespread inflammatory diseases.
- Intrathecal Administration: Injection into the cerebrospinal fluid (CSF); effective for central nervous system and neurological conditions.
- Direct/Localized Injection: Injection directly into the damaged tissue (e.g., knee or heart); ensures maximum cell concentration at the target site.
- Intranasal Administration: Non-invasive route through the nasal cavity; emerging for neurological conditions.
Safety Profile and Side Effects of Stem Cell Therapy
Stem cell therapy shows a favorable safety profile across hundreds of clinical trials, particularly when using properly isolated cells.
Common Short-Term Side Effects
Most side effects are mild and temporary, resolving quickly. These include fatigue, headache, chills after IV administration, or localized discomfort and swelling after direct injection. These are generally manageable with standard rest and over-the-counter pain relievers.
Long-Term Safety Considerations
Safety data from over 1,200 patients treated with pluripotent stem cell products show no generalizable class-wide safety concerns.
- Tumor Risk: This theoretical risk, particularly with pluripotent stem cells, appears very low in clinical trials using properly differentiated cells. The risk remains highest for ESCs.
- Immune Reactions: Autologous therapies (using the patient’s own cells) eliminate rejection risk. Allogeneic MSC therapies have low immunogenicity, meaning they are well-tolerated.
Distinguishing Safe from Unsafe Clinics
Unregulated clinics pose serious patient safety risks. Patients must be vigilant:
- Red flags include making guaranteed cure claims, lacking proper FDA oversight, using unsterile procedures, and providing no transparent follow-up.
- Reputable clinics are FDA-registered, publish clinical trial data, use strict manufacturing protocols, and employ highly trained medical professionals who discuss both benefits and risks realistically.
Stem Cell Therapy vs. Traditional Treatments: A Comparative Analysis
Stem cell therapy (a regenerative medicine approach) differs fundamentally from traditional treatments (a palliative approach).
| Factor | Stem Cell Therapy | Traditional Treatment | Key Difference |
| Mechanism | Repairs/regenerates tissue (addresses root cause). | Manages symptoms (doesn’t repair damage). | Stem cells are potentially curative; traditional treatments are palliative. |
| Invasiveness | Minimally invasive (injection-based cellular therapy). | Varies: Injections to highly invasive surgery. | Often less invasive than surgery. |
| Recovery Time | Hours to days. | Varies: Days to many months (surgery). | Recovery is typically much faster. |
| Long-term Durability | Potentially lasting (cells keep working). | Effects fade as medication clears. | Stem cells may provide more sustained benefit. |
For conditions like heart failure, stem cell treatment has proven significantly more effective than conventional therapy in improving heart function (LVEF improvement) and reducing hospitalization rates (60% lower). The initial cost of stem cell therapy may be high ($5,000–$30,000), but long-term cost-effectiveness is substantial due to reduced hospitalizations and less need for chronic medication.
The Future of Stem Cell Therapy: Emerging Technologies and Anticipated Developments
The field of regenerative medicine is rapidly advancing:
- iPSC Expansion: Focused on creating scalable, personalized cellular therapy using genetically enhanced, “off-the-shelf” iPSC-derived cell products that don’t cause rejection.
- Organ Regeneration: Advancing the goal of creating functional organs through 3D bioprinting and developing vascularized tissues, moving closer to future organ replacement.
- Exosome Therapies: Isolating exosomes (tiny therapeutic molecules secreted by stem cells) for targeted delivery, simplifying treatment, and offering sophisticated drug delivery systems.
Experts predict that within five to ten years, stem cell-based therapies will be commonly prescribed for numerous conditions beyond blood diseases, reflecting the rapid pace of regulatory approval and scientific advancement.
Making an Informed Decision: Questions to Ask Your Healthcare Provider
If you’re considering stem cell therapy, asking the right questions is critical for making an informed decision about your cellular therapy.

About Your Condition and Treatment Options
- Is my condition FDA-approved for stem cell therapy, or is it a clinical trial?
- What is the expected timeline for improvement, and what realistic outcomes should I anticipate?
- What research supports the effectiveness of stem cell therapy for my specific condition?
About the Stem Cell Therapy Protocol
- What type of stem cells will be used (autologous, allogeneic, iPSC-derived)?
- Where will the cells be harvested, and what risks are involved in that procedure?
- How will the stem cells be administered (IV, localized injection, other)?
Red Flag Responses to Avoid
Be wary of any provider that offers guaranteed cure promises for chronic diseases, lacks proper FDA oversight, is unwilling to discuss risks, or charges extremely high fees without transparent protocols. Always look for published clinical trial data and realistic expectations.
Take the Next Step: Consult and Connect
Stem cell therapy represents a genuine frontier in regenerative medicine, offering hope for conditions where traditional medicine has limitations. If you suffer from a chronic condition, degenerative disease, or injury that hasn’t responded to conventional treatments, stem cell therapy may offer new possibilities.
As a commitment to providing accessible regenerative medicine, the Nova Voya platform is proud to partner with top clinics to offer FDA-compliant Stem Cell Therapy protocols, including advanced MSC treatments, at a special, value-based price. Contact the Nova Voya platform today to learn about these exclusive patient options and begin your personalized consultation.
General Next Steps:
- Consult with qualified professionals: Discuss stem cell therapy options with your physician and ask for referrals to centers conducting FDA-regulated clinical research.
- Evaluate your options carefully: Compare stem cell therapy with other available treatments, considering efficacy, safety, cost, and the required timeline.
Have you or someone you know considered stem cell therapy for a medical condition? What factors influenced that decision? Was it the potential for tissue regeneration or the hope for a long-term fix? Share your thoughts, experiences, or questions in the comments below. Your perspectives help others navigate similar health decisions and contribute to the conversation around this rapidly evolving field of medicine.
Conclusion: Stem Cell Therapy’s Promise and Reality in Modern Medicine
Stem cell therapy has evolved from concept to clinical reality, offering genuine hope for chronic conditions. This regenerative medicine approach focuses on fundamental repair and tissue regeneration, leveraging the body’s intrinsic healing capacity. The overall safety profile is favorable, and success rates range from 50% to 90% across mature applications like HSCT and MSC therapy. As iPSC technology matures, stem cell treatment is expected to become commonplace within the next decade. Patients must work exclusively with established, FDA-regulated medical institutions to ensure safety and meaningful outcomes.
Frequently Asked Questions
Yes, it is legal when administered in licensed clinics for approved medical uses. However, unlicensed or unproven treatments are not permitted.
Initially, subtle improvements appear within days to weeks. Meaningful, measurable results typically emerge within 3–6 months as cells integrate and promote tissue regeneration.
Not necessarily. Autologous cells (the the patient’s own) eliminate the risk of rejection. Allogeneic cells (donors), like MSCs, are immediately available but carry minor immune risks.
Reputable clinics are FDA-registered, affiliated with academic centers, use rigorous quality control, publish clinical outcomes, and avoid guaranteed cure claims.
It offers long-lasting improvement and remission, but “cure” varies. For blood disorders (HSCT), a cure is possible; for degenerative diseases, it slows progression and substantially reduces symptoms for years.